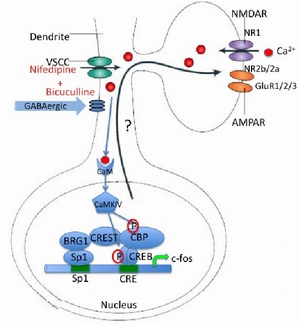
Qiu, Zi-Long 仇子龙 Ph.D. zqiu@@ion.ac.cn 仇子龙: 1994-1998年就读于上海交通大学生物科学与技术系,获学士学位。1998-2003 年就读于中国科学院上海生命科学院生物化学与细胞生物学研究所,获博士学位。2003-2009年在美国加州大学圣迭戈分校神经生物学系从事博士后研究工 作。2009年受聘于中国科学院上海生命科学研究院神经科学研究所任研究员,课题组长。 Zilong Qiu was born in Beijing and grew up in Anhui, China. From 1994-1998, he attended Shanghai Jiao Tong University and graduated with a BS in Biological Sciences. He was a graduate student with Dr. Kan Liao from 1998-2003 at the Shanghai Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences. In his Ph.D. thesis, he focused on the molecular mechanism of adipocyte differentiation. With this molecular biology and cell signalling background, he became eager to explore the field of molecular neuroscience during the last several years as a graduate student and came to Dr. Anirvan Ghosh’s Lab at University of California, San Diego to pursue this dream. His postdoctoral project focuses on activity dependent transcriptional regulation of genes in the rodent cerebral cortex. He joined ION faculty as Principle Investigator from July, 2009. 大脑是动物用于感知和适应环境的重要器官。面对千变万化的环境,大脑必须能够记录有用的信息,摒除无关的信息,并 针对感觉输入和外部环境做出各种决定。外界信息通过电活动的方式被感觉器官迅速传递至大脑,为什么毫秒级的电活动往往可以对大脑的信息储存产生长时间的影 响?现在认为,是因为神经细胞之间的电信号在神经细胞内部被转换为化学信号,通过第二信使激活各种胞内信号通路,被激活的信号分子最终进入细胞核,作用于 基因表达开关,导致基因组上的基因被选择性的打开或关闭,进而影响到神经细胞内的相应基因编码蛋白质的水平。这样,神经电活动通过调控基因表达的方式,就 可以对神经细胞的生理活动产生影响,从而最终修饰神经网络而影响大脑的生理功能。 神经细胞网络会根据输入信息对自身的连接和电生理活性进行调节的过程被称为,神经可塑性。正是因为神经系统的功能可塑, 大脑才能快速的对外界刺激产生反应,更适应外界的刺激或者长期储存重要的信息。我们认为神经电活动诱导基因表达的过程,对神经可塑性的过程有重要贡献。已 经有很多证据证明,这个过程的阻断,会对各种神经可塑性的发展以致大脑的各种生理功能产生严重的影响。 神经电活动调控基因表达的过程对大脑的重要还表现在,已经发现多种神经系统遗传疾病的发生与此过程有关。比如属于自闭症 (Autism spectrum disorders) 范畴的瑞特综合症 (Rett syndrome) 的产生就是一个神经电活动调控的转录抑制因子-甲基化DNA结合蛋白MeCP2-的突变所致。这种遗传疾病提示我们,如果神经电活动调控基因表达的过程发 生异常,大脑的正常发育过程会被严重破坏。自闭症的发生发展还完全没有被人们从分子和细胞水平进行认识。究竟这个过程如何发生,也是我们感兴趣的问题。 我们研究组采用分子细胞生物学,小鼠遗传学和电生理学等多种途径研究这个神经电活动诱导基因表达的过程在分子水平,细胞水平,神经细胞网络水平和整体动物水平怎样修饰神经网络,对神经系统的可塑性产生影响,而最终怎样对动物的行为发生作用。 我们正在开展的课题有,1.寻找神经电活动调控基因表达的分子开关及确认神经电活动调控的靶基因 2.对靶基因的功能研究。这些被神经电活动调控的靶基因究竟怎样影响神经细胞的生理功能和怎样影响神经网络的可塑性 3.MeCP2介导的神经电活动调控基因表达过程如何对神经可塑性以及大脑发育产生影响,以及为什么MeCP2的基因突变会导致自闭症范畴的神经系统疾 病。我们希望通过基础研究对神经系统疾病的最终治疗做出贡献。 Research Interest One of the most remarkable features of the nervous system is that its structure and function can be modified by sensory input, the research interest of our laboratory is to address mechanisms underlying this process from molecular level. It is now well established that neuronal activity plays a critical role in controlling many aspects of neural development and function, including neuronal viability, migration, morphogenesis, and plasticity (reviewed in Katz and Shatz, 1996). The effects of activity in the nervous system are primarily mediated by calcium signaling. Calcium influx leads to post-translational modification of synaptic proteins as well as induction of new gene expression. The lasting effects of neuronal activity, such as activity-dependent dendritic growth, long-term plasticity in sensory systems, and memory consolidation, require calcium-dependent transcription (reviewed in Flavell and Greenberg, 2008).
During the last two decades we have learned a great deal about the molecular mechanism by which the CREB-associated complex activates gene expression upon calcium influx through glutamate receptors and voltage sensitive calcium channels, which laid the foundation for our understanding of the molecular basis of cognitive behavior (reviewed in Qiu and Ghosh, 2008). Despite these advances, knowledge of how protein products of CREB-dependent transcription contribute to synaptic functions remains very limited. The question of how calcium-dependent gene expression contributes to neural plasticity and cognition has yet to be answered. Calcium RESponsive Transactivator (CREST) was recently identified as a transcriptional regulator of calcium dependent gene expression in a Transactivator Trap screen in Dr. Anirvan Ghosh’s lab (Aizawa et al. 2004). During his postdoctoral training, Dr. Qiu identified a CREST-containing transcriptional complex and discovered a novel calcium-dependent regulatory mechanism. Importantly, he found that expression of several glutamate receptor genes is regulated by the CREST complex and calcium signaling, which suggests that CREST may contribute to calcium-dependent neural plasticity and cognitive behavior. In future work, the laboratory is interested in addressing the function of CREST on neural plasticity and cognitive behavior in rodents, using molecular biology, electrophysiology and mouse genetics approaches (Qiu and Ghosh, 2008). Recent studies suggest that activity-dependent adaptive mechanisms, including gene expression, are also implicated in neurological disorders, such as autism spectrum disorders (Morrow et al. 2008). In previous work, Dr. Qiu discovered that a methyl-DNA binding protein-MeCP2 is regulated by the CREST complex. Mutation of MeCP2 gene causes Rett syndrome, one of the most severe autism spectrum disorders affecting child development (reviewed in Chahrour and Zoghbi, 2007). In future research, the laboratory is interested in studying the relationship between calcium-dependent gene expression and neuropathology of autism spectrum disorders. We believe that these studies will greatly improve our knowledge of the molecular basis of cognitive and social behavior, as well as contribute to possible therapeutic treatments for autism spectrum disorders.代表性论文: 1. Qiu, Z., Sylwestrak, E., Ghosh, A., The Rett syndrome protein MeCP2 regulates synaptic scaling. (Submitted) 2. Qiu, Z. (2009) Histone modifier, the gatekeeper of good memory. Cell Res. 8: 920-921 (Research Highlight) 3. Qiu, Z., and Ghosh, A. (2008). A Calcium-dependent switch in a CREST-BRG1 complex regulates activity-dependent gene expression. Neuron 60: 775-787. (Highlighted in Molecular Biology Select of Leading Edge section on Cell at Jan. 29. 2009) 4. Qiu, Z., and Ghosh, A. (2008). A brief history of neuronal gene regulation: regulatory mechanisms and cellular consequences. Neuron 60: 449-455. (Invited review) 5. Yuan, S., Qiu, Z., and Ghosh, A. (2008). Tox3: a CREB- and CBP-interacted protein that regulates calcium-dependent transcription in neurons. Proc. Natl. Acad. Sci. USA. 106: 2909-2914. 6. Wu, J. I., Lessard, J., Olave, I. A., Qiu, Z., Ghosh, A., Graef, I. A., and Crabtree, G. R., (2007). Regulation of dendritic development by neuron-specific chromatin remodeling complexes. Neuron 56: 94-108. 7. Kashani, A. H., Qiu, Z., Jurata, L., Lee, S. K., Pfaff, S., Goebbels, S., Nave, K. A., and Ghosh, A. (2006). Calcium activation of the LMO4 transcription complex and its role in the patterning of thalamocortical connections. J. Neurosci. 26: 8398-8408. 8. Qiu, Z., Wei, Y., Chen, N., Jiang, M., Wu, J., and Liao, K. (2001). DNA synthesis and mitotic clonal expansion is not a required step for 3T3-L1 preadipocyte differentiation into adipocytes. J. Biol. Chem. 276: 11988-11995. 9. Qiu, Z, Guo, X. M. and Liao, K. (2001). Analysis of the Relationship between Globally Regulated Genes and Their Chromosomal Genetic Map Loci during Adipogenesis. Sheng Wu Hua Xue Yu Sheng Wu Wu Li Xue Bao (Shanghai). 33: 137-141. 10. Jin, S., Zhai, B., Qiu, Z., Wu, J., Lane, M. D., and Liao, K. (2000). c-Crk, a substrate of the insulin-like growth factor-1 receptor tyrosine kinase, functions as an early signal mediator in the adipocyte differentiation process. J. Biol. Chem. 275: 34344-34352. 11. Tang, W. H, Qiu Z, Wang, Z, Zhang, J and Hong, M. M. (1999). ACTA PHYTOPHYSIOLOGICA SINICA 25: 401-407. (责任编辑:泉水) |
仇子龙 研究员
时间:2009-12-16 10:01来源:ion.ac.cn 作者:admin
顶一下
(5)
83.3%
踩一下
(1)
16.7%
------分隔线----------------------------
- 发表评论
-
- 最新评论 进入详细评论页>>
- 推荐内容
-
- Jun Yang,Ph.D.
un Yang, Ph.D. Assistant Professor in Ophthalmology and Visual Sciences Adjunct...
- 仇子龙 研究员
Qiu, Zi-Long 仇子龙 Ph.D. zqiu@@ion.ac.cn 仇子龙: 1994-1998年就读于上海...
- 李澄宇 研究员
Li, Cheng-Yu, Ph.D. Investigator tonylicy@ion.ac.cn 李澄宇博士,1995-1999年就...
- 俞立受聘清华大学生物系教授
俞立 俞立1972年生,2008年9月回国工作,任生物系教授。1994年获...
- 段树民教授简介
段树民 中国科学院神经科学研究所研究员。博士生导师,中国科...
- 朱剑虹教授简介
朱剑虹 教授简介:1955 年11 月生,汉族,江苏南京人,中共党员...
- Jun Yang,Ph.D.