|
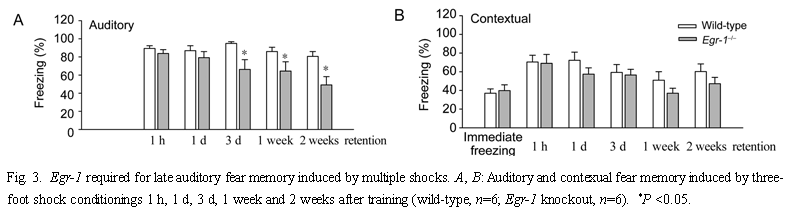
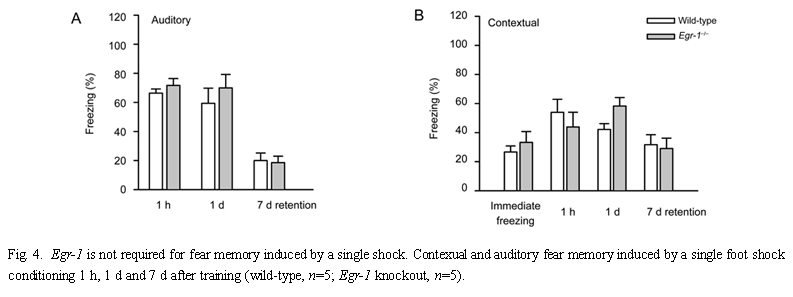
Since the defect in fear memory was not apparent until later time points after training with multiple shocks, we wanted to test if fear memory induced by a single shock-tone pairing would also be altered. Neither contextual nor auditory fear memory was significantly different in Egr-1 knockout mice when compared to wild-type mice [F(1,24)=0.3, P=0.6, for context; F(1,24)=0.8, P=0.4, for auditory] (n=5, Fig.4). Both knockout and wild-type mice showed a significant reduction in the freezing response by 7 d after training (P<0.05 for both, 1 h vs 7 d, Fig.4B). This indicates that Egr-1 may not contribute to fear memory induced by a single shock-tone pairing. 2.3 Trace fear memory To investigate if Egr-1 is truly selective for amygdalar (vs hippocampal) fear memory, we tested Egr-1 knockout and wild-type mice in the "trace" memory paradigm. Trace memory has recently been shown to be hippocampal-dependent, NMDA receptors in the hippocampus were shown to be important for the formation of memories that associate events across time[50]. Previous studies showed that Egr-1 contributes to NMDA receptor dependent hippocampal synaptic LTP in the CA1 and dentate gyrus regions[41,42]. Therefore, Egr-1 may contribute to fear memory induced by the hippocampal-dependent, trace fear-conditioning paradigm. We found significant fear memory induced by this 'trace' training paradigm in wild-type mice [compare (3.3±1.6) % freezing before training to (71.7±6.9)% freezing 1 d after training, P<0.001, n=5, Fig.5]. Three days after training, the freezing responses of mice trained under the trace paradigm were comparable to those who had received multiple paired training [compare (59.3±8.4)% freezing with (58.9+11.8)% freezing after trace conditioning on day 3]. We found no significant difference in contextual trace fear memory between wild-type and Egr-1 knockout mice (n=7) [F(1,50)=1.5, P=0.2] 1, 3 d, 1 and 2 weeks after training. Two weeks after training, both groups retained good trace fear memory [compare (52.8± 5.6)% vs (54.8±6.2)% freezing, wild-type vs knockout on day 14]. This finding clearly indicates that Egr-1 is not required for trace fear memory.
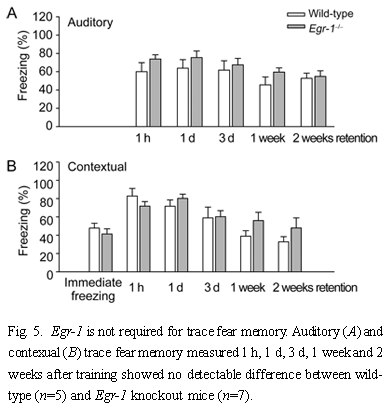
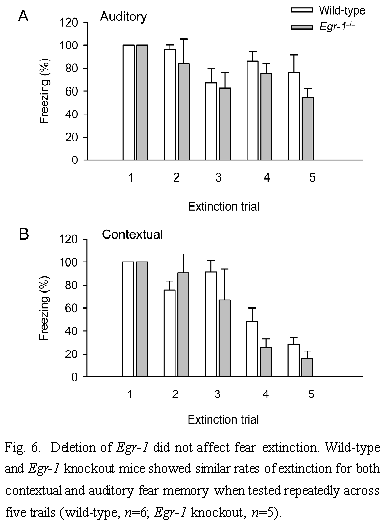
2.4 Fear extinction Fear extinction is an active learning process that occurs when a conditioned stimulus is repeatedly nonreinforced[52]. To determine if Egr-1 knockout mice display significant changes in the extinction of contextual and auditory fear memory, we measured fear responses for five trails at 1 h intervals. Mice were trained with three shock-tone pairings as described in Fig.3. We normalized the percentage change in fear memory to control responses and found no significant difference between Egr-1 knockout (n=5) and wild-type mice (n=6) in the extinction of either contextual or auditory fear memory [F(1,45)=1.4, P=0.2 for context, F(1,45), P=2.0 for auditory] (Fig.6). These results suggest that the deficit in long-term auditory fear memory is not due to extinction of the fear response but due to repeated testing.
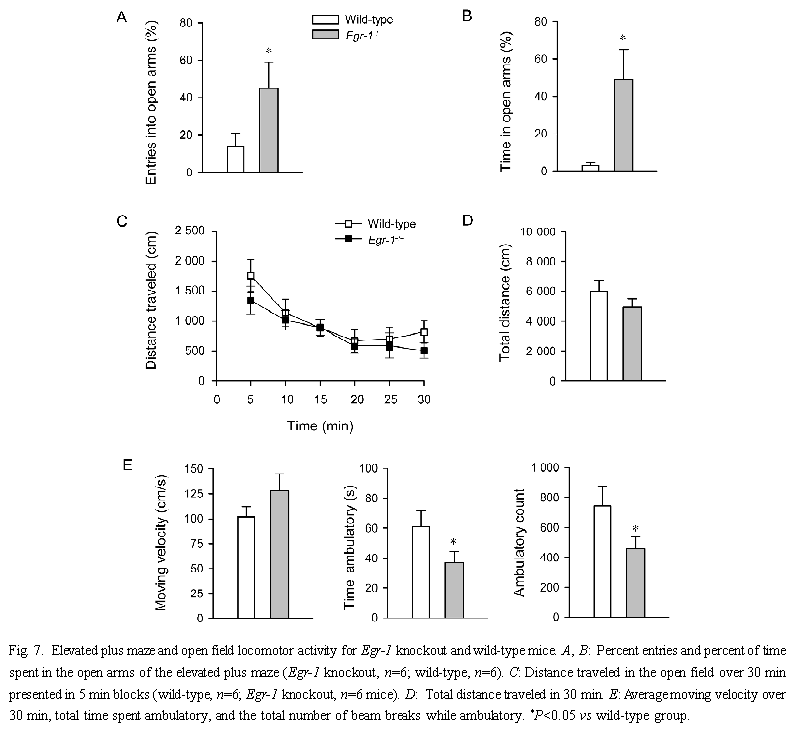
2.5 Elevated plus maze and open field activity To determine if anxiety-related behaviors were altered in Egr-1 knockout mice, we measured their performance in the elevated-plus maze. The number of entrances and total time spent in the open arms were used as indicators of anxiety-like behavior. As shown in Fig.7A, Egr-1 knockout mice showed a significant increase in the total number of entrances into the open arms (n=6) as compared with wild-type mice [n=6, t(11)=1.82, P<0.05]. Additionally, the total time spent in the open arms was significantly increased in Egr-1 knockout mice, as compared with wild-type mice [t (11) = 2.68, P<0.05, Fig.7B]. However, the total entrances into the open arms and closed arms were not significantly different between wild-type and Egr-1 knockout mice, indicating that this finding is not simply a result of general hyperactivity. We also evaluated the general locomotor behavior of both wild-type and Egr-1 knockout mice in a novel open field (n=6 for each group). There was no difference between Egr-1 knockout and wild-type mice in the total distance traveled and the mean moving velocity, indicating that the deletion of Egr-1 did not affect general locomotor behavior (Fig.7C, D). However, the time spent ambulatory and ambulatory counts in Egr-1 knock out mice were significantly smaller than that of wild-type mice [t (10) = 1.87, P<0.05; t (10) = 1.81, P<0.05, Fig.7E].
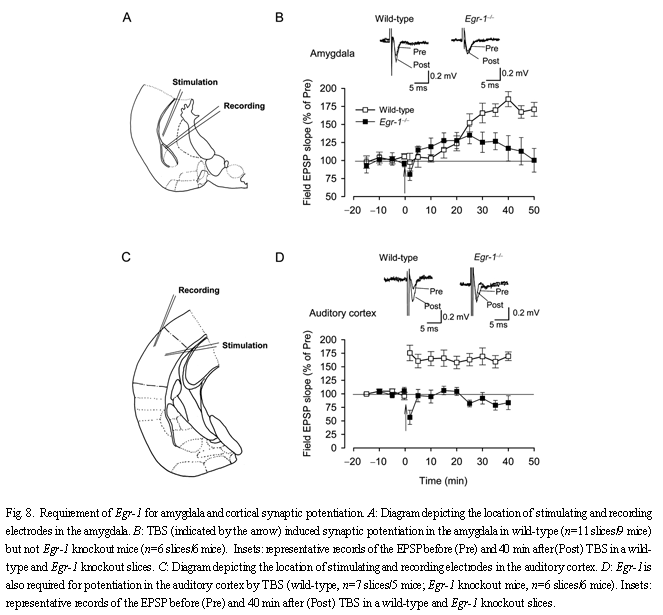
2.6 Fear memory-related synaptic plasticity Synaptic plasticity, such as long-term potentiation (LTP), is thought to be important for learning and memory. Here we wanted to examine synaptic potentiation in the amygdala, a structure known to play an important role in fear memory[53,54]. We examined synaptic potentiation at 'thalamic' input synapses to the lateral amygdala by placing a stimulating electrode in the ventral striatum[55]. Based on our previous studies[56], we used five trains of theta burst stimulation (TBS). In slices of wild-type mice, TBS induced significant synaptic potentiation [(n=9 slices/8 mice; (169.4±8.0)%; Fig.8B]. However, synaptic potentiation in slices of Egr-1 knockout mice was significantly reduced or blocked [n=6 slices/6 mice; (124.1±14.1)%, t(13)=2.4, P<0.05 compared with slices of wild-type mice]. We also examined synaptic potentiation in the auditory cortex. In slices of both wild-type and Egr-1 knockout mice, similar baseline field EPSPs were induced by local stimulation (see samples in Fig.8). In the auditory cortex, TBS induced a long-lasting enhancement of synaptic responses persisting for at least 40 min after induction [n=7 slices/6 mice, (150.2±12.6)%]. In Egr-1 knockout mice, however, synaptic potentiation was abolished [n=8 slices/8 mice, (81.9±10.0)%; P<0.05 compared with potentiation in wild-type mice] (Fig.8D). These results provide strong evidence that Egr-1 contributes to synaptic potentiation in these cortical areas, and that Egr-1 contributes to plasticity in both the amygdala as well as the auditory cortex.
3 DISCUSSION Our results provide evidence for a role of Egr-1 in anxiety-related behavior and long-term auditory fear memory. These findings are in good accord with previous reports that show Egr-1 was activated by associative fear conditioning[57]. More interestingly, Egr-1 plays a selective role in late, but not early, behavioral responses to auditory fear memory. Early auditory fear memory, measured 1 h or 1 d after conditioning, was not affected in Egr-1 knockout mice while fear responses were significantly decreased starting 3 d after conditioning (Fig.3). Fear memory generated by the single shock conditioning paradigm and contextual memory were similar between knockout and wild-type mice (Fig.3 and 4). Additionally, Egr-1 knockout mice did not differ from wild-type mice in fear memory using the 'trace' memory paradigm[50](Fig.5). Egr-1 knockout mice showed no significant defects in several sensory and motor tests; such as acute pain threshold measurements[58] and open field motor activity (Fig.7), therefore we argue that changes in fear memory are unlikely to be due to general developmental defects. Due to the use of Egr-1 knockout mice without regional selectivity, it is not possible for us to dissect the role of Egr-1 in particular brain regions. Different parts of the amygdala have been shown to contribute to behavioral fear responses (e.g., lateral vs basal amygdala)[3,59,60]. In the hippocampus, Egr-1 is up-regulated by tetanic stimulation[33-36], behavioral learning, or memory retrieval[37-39]. Deletion of Egr-1 leads to a reduction or blockade of hippocampal LTP in the CA1 region and dendate gyrus[41,42], as well as an impairment of spatial memory[42]. Based on these results, the genetic deletion of Egr-1 should at least reduce hippocampus-dependent memory tasks such as trace fear memory and contextual fear memory. In the present study, however, we found that contextual fear memory and trace fear memory are intact in Egr-1 knockout mice, although late auditory fear memories were significantly reduced. There are at least a few possible explanations for our findings. First, the functional role of Egr-1 in hippocampus-dependent memory may be compensated by alternative signaling pathways. Future studies using inducible knockout mice for Egr-1 may help to test this possibility. Second, it is possible that hippocampal-dependent functions may be compensated by neuronal interactions between the hippocampus and its related central structures or simply by other central nuclei in Egr-1 knockout mice. (责任编辑:泉水) |
转录因子Egr-1参与长期性恐惧记忆和焦虑(3)
时间:2006-03-25 16:27来源:Acta Physiologica Sinica 作者:bioguider
顶一下
(25)
100%
踩一下
(0)
0%
------分隔线----------------------------
- 发表评论
-
- 最新评论 进入详细评论页>>
- 推荐内容
-
- 中科院神经所自2002年以来所发表的
所有数据都来自于 www.scholar.google.com 。虽然 GOOGLE 半年为文章的...
- 中科院神经所自2002年以来所发表的