|
是什么让吗啡等阿片类镇痛药物药效降低、产生耐药性,甚至令人嗜药成瘾?在刚刚出版的国际权威学术期刊Cell (《细胞》)上,公布了中科院上海生命科学研究院神经所张旭博士重大发现:P物质是“元凶”。 |
传统理论认为,存在于脊髓中的P物质作用仅是致痛,其实它干的“坏事”远不止此。研究组组长、中科院上海生命科学研究院神经科学研究所张旭博士介绍,人体内的阿片受体在感受痛觉的神经元表面严阵以待,专门执行麻醉剂的镇痛指令。其中 “mu”的受体发挥镇痛作用,而P物质却带领“delta”受体找到前者,降低镇痛作用,同时增加人体对镇痛剂的耐药性。因此,P物质可以称为是是调控吗啡镇痛的“关键人物”。
没有了P物质,添乱的delta阿片受体就没了“领路者”,找不到mu阿片受体,自然也就无法影响药效,无法产生耐药性等副作用。研究人员在小鼠身上做实验,去除其体内的P物质基因,使其无法分泌P物质,结果欣喜发现:小鼠对吗啡不再产生耐受,注射次数再多,药效也没有下降。
此次发现P物质是直接调控阿片系统镇痛功能和吗啡耐受的罪魁祸首,将为今后开发药效强、副作用小的新型镇痛药提供全新理论基础。
张旭博士在接受记者采访时说,此次新发现突破了以往痛觉调控系统的传统认识,也给临床治疗提供了启发:也就是说,只要抑制人类的P物质基因,就有希望开发出全新止痛药——只镇痛、不“耐受”,效果持之以恒,而不必加大用量。但他同时表示,该成果只能解决吗啡类药物的耐受性,但无法阻止“上瘾”副作用。
Figure 1. Requirement of Preprotachykinin A Expression for LDCV Localization of DOR in Small DRG Neurons
(A) Double-immunofluorescence labeling shows colocalization of DOR (in green) and substance P (SP, in red) in LDCVs in small DRG neurons of the mouse. Pre-embedding immunogold-silver labeling of DOR combined with postembedding immunogold (10 nm in diameter) labeling of substance P shows localization of DOR (arrowhead) in the membrane of LDCV and substance P (arrow) in the lumen of LDCV. Quantitative analysis at the ultrastructural level shows that the LDCVs carrying DOR almost exclusively contain substance P (a total of 180 LDCVs in three experiments). Data are represented as mean ± SEM.
(B) Double-immunofluorescence labeling shows that DOR (in green) and CGRP (in red) are colocalized in LDCVs in small DRG neurons of wild-type (+/+) mice but not in LDCVs of PPT-A knockout (−/−) mice. The number of CGRP-containing vesicles in small DRG neurons of PPT-A knockout mice is unchanged (for both +/+ and −/− neurons, 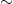 7 LDCVs/μm2 in the optic section through the center of neuron).
7 LDCVs/μm2 in the optic section through the center of neuron).
(C) In small DRG neurons of PPT-A knockout mice, immunogold-silver labeling of DOR is seen in multivesicular bodies (arrowhead) instead of in LDCVs (mean ± SEM, n = 18 neuron profiles). **p < 0.01 versus the neurons of wild-type mice. Postembedding immunogold labeling shows that, in small DRG neurons of PPT-A knockout mice, CGRP is still localized in LDCVs (arrowhead).
Scale bars, 5 μm for confocal images; 100 nm for electron micrographs.
原文出处:
Guan, J., Xu, Z., Gao, H., He, S., Ma, G., Sun, T., Wang, L., Zhang, Z., Lena, I., Kitchen, I., Elde, R., Zimmer, A., He, C., Pei, G., Bao, L. and Zhang, X. (2005) Interaction with vesicle luminal protachykinin regulates surface expression of δ-opioid receptors and opioid analgesia. Cell 122: 619-631. [Abstract] [PDF]
张旭博士及其实验室简介

Xu Zhang, Ph.D.
Rm 219, ION Building
Institute of Neuroscience
Chinese Academy of Sciences
Shanghai 200031, China
Email: xu.zhang@@ ion.ac.cn
lab homepage
实验室成员:
[NextPage]

Dr. Xu Zhang graduated from Fourth Militory Medical University in Xi'an, China in 1985, and obtained his Ph.D. at Department of Neuroscience, Karolinska Institute, Sweden in 1994. He joined ION in 1999 and is now an investigator and head of the Laboratory of Sensory System. His main interest is to study the molecular and cellular mechanisms involved in the regulation of sensory signaling in the dorsal root ganglion neurons and spinal dorsal horn neurons, especially the mechanisms of chronic pain and nerve regeneration following peripheral nerve injury.
Research Interests
We are interested in the basic mechanisms underlying gene activation and the sorting, transport and targeting of proteins in sensory neurons. Our long-term research goal is to understand the neurobiological basis of chronic pain and nerve regeneration and to identify rational therapeutic approaches in curing disfunctions in human sensory systems.
From gene regulation and protein trafficking to neuronal modulation and neuropathic pain. Neuropeptides play important roles in the transmission and modulation of pain sensation. We are thus interested in the gene regulation, processing and trafficking of neuropeptides and their receptors in sensory neurons under physiological or pathological conditions. Since the distribution of membrane receptors and ion channels in the axons and dendrites are susceptible to modulation and such modulation has direct consequences in the function and plasticity of synaptic transmission, we are focusing on the correlation between the changes in the distribution of receptors and ion channels in the pre- and postsynaptic membranes and the functional modulation of primary sensory neurons and their synaptic properties. For examples, peripheral nerve injury and inflammation cause dramatic changes in the functions of dorsal root ganglion (DRG) neurons, often leading to chronic pain. We have thus focused our studies on the plasticity of DRG and spinal cord neurons after peripheral nerve injury and inflammation, e.g., changes in gene expression, cellular localization and functions of neuropeptides and their receptors, transmitters and their receptors, as well as ion channels and related channel modulators. In addition, we are also examining changes in physiological and anatomical changes in the neural circuits in the dorsal horn of the spinal cord in animal models of neuropathic and inflammatory pain.
Ongoing Projects
Long-term plasticity of DRG neurons at the level of gene expression.
To determine global alterations in neuronal gene expression following nerve injury, we have carried out cDNA array analysis on cDNA libraries of lumbar DRGs of normal rats and of rats 14 days after peripheral axotomy. Of 7523 genes and expression sequence tags (ESTs) examined, marked alteration in the expression of 122 genes and 51 ESTs was found. These genes encompass a large number protein families, including neuropeptides, membrane receptors, ion channels, signal transduction molecules, and synaptic vesicle proteins. Of particular interest is the upregulation of ¦Ã-aminobutyric acidA receptor ¦Á5 subunit, peripheral benzodiazepine receptor, nicotinic acetylcholine receptor ¦Á7 subunit, P2Y1 purinoceptor, sodium channel ¦Â2 subunit and calcium channel ¦Á2¦Ä-1 subunit. Our finding reveals a dynamic and complex change in the gene expression in DRG neurons after peripheral axotomy. We are also examining changes in the gene expression of dorsal horn neurons using similar cDNA array analysis.
Long-term structural plasticity in DRG and spinal cord.
Peripheral axotomy-induced sprouting of thick myelinated afferents (A-fibers) from laminae III-IV into laminae I-II of the spinal cord has been postulated to be the structural basis of neuropathic pain. However, our recent study showed that axotomy-induced sprouting from deeper to superficial layers is much less pronounced than previously assumed. This conclusion is based on our labeling studies using cholera toxin B subunit (CTB), a neuronal tracer used to demonstrate the sprouting of A-fibers in several earlier studies. We found that CTB could label unmyelinated afferents (C-fibers) in lamina II and thin myelinated afferents in lamina I, when it is applied after peripheral axotomy. In an attempt to label large DRG neurons and A-fibers selectively, CTB was applied four days before axotomy ('pre-injury-labeling'), and sprouting was monitored after axotomy. We find that only a small number of A-fibers sprouted into inner lamina II, a region normally innervated by C-fibers, but not into outer lamina II or lamina I. Neuropeptide Y (NPY) is found in these sprouts in inner lamina II, an area very rich in Y1 receptor-positive processes. Although limited in numbers, this large NPY immunoreactive DRG axon sprouting into the inner lamina II may account for a functional circuitry involved in neuropathic pain.
Short-term plasticity DRG and spinal cord neurons.
Neurotransmitter receptors and ion channels are important for the regulation of pain transmission and pain treatment. Our study is focused on the cellular and molecular mechanisms involving in the trafficking of membrane receptors and channels in primary sensory neurons and the dorsal spinal cord neurons, and the regulation and changes in trafficking mechanisms under physiological and pathological conditions. We believe that the sensitivity of the neurons can be modified by changing the plasma membrane composition of receptors and channels within the time scale of seconds to minutes in response to different extracellular stimuli.
Publications Guan, J., Xu, Z., Gao, H., He, S., Ma, G., Sun, T., Wang, L., Zhang, Z., Lena, I., Kitchen, I., Elde, R., Zimmer, A., He, C., Pei, G., Bao, L. and Zhang, X. (2005) Interaction with vesicle luminal protachykinin regulates surface expression of δ-opioid receptors and opioid analgesia. Cell 122: 619-631. PDF
Zhang, K., Xiao, H., Lu, P., Shi, J., Li, G., Wang, Y., Han, S., Zhang, F., Lu, Y., Zhang, X., and Xu., X. (2004) Differential Gene Expression after Complete Spinal Cord Transection in Adult Rats: An Analysis Focused on a Subchronic Post-Injury Stage. Neurosci. 128: 375-388.PDF
Guan, J., Xu, Z., Gao, H., He, S., Ma, G., Sun, T., Wang, L., Zhang, Z., Lena, I., Kitchen, I., Elde, R., Zimmer, A., He, C., Pei, G., Bao, L. and Zhang, X. (2005) Interaction with vesicle luminal protachykinin regulates surface expression of δ-opioid receptors and opioid analgesia. Cell in press.
Yang, L., Zhang, F., Huang, F., Lu, Y., Li, G., Bao, L., Xiao, H., and Zhang, X. (2004) Peripheral nerve injury induces trans-synaptic modification of channels, receptors and signal pathways in rat dorsal spinal cord. Eur. J. Neurosci. 19, 871-883.PDF
Xu, N., Bao, L., Fan, H., Bao, G., Pu, L., Lu, L., Wu, C., Zhang, X., and Pei, G.(2003) Morphine Withdrawal Increases Glutamate Uptake and Surface Expression of Glutamate Transporter GLT1 at Hippocampal Synapses. J. Neurosci., 23: 4775-4784.PDF
Bao, L., Jin, S., Zhang, C., Cai, H., Xu, Z., Wang, L., Xiao, H., Ning, F., Zhang, F., Lu, Y., Hokfelt, T., Zhou, Z., and Zhang, X. (2003) Activation of delta-opioid receptors induces receptor insertion and neuropeptide secretion. Neuron, 37,121-133PDF
Bao, L., Wang, H., Cai, H., Tong, Y., Jin, S., Lu, Y., Grant, G., Hokfelt, T., and Zhang, X.(2002) Peripheral axotomy induces only very limited sprouting of coarse myelinated afferents into inner lamina II of rat spinal cord. Eur. J. Neurosci. 16: 175-185PDF
Li, G., Wo, Y., Zhong, M., Zhang, F., Bao, L., Lu, Y., Huang, Y., Xiao, H., and Zhang, X. (2002) Expression of fibroblast growth factors in rat dorsal root ganglion neurons and regulation after peripheral nerve injury. NeuroReport 13: 1903-1907 PDF
Xiao, H., Huang, Q., Zhang, F., Bao, L., Lu, Y., Guo, C., Yang, L., Huang, W., Fu, G., Xu, S., Cheng, X., Yan, Q., Zhu, Z., Zhang, X., Chen, Z., Han Z., and Zhang, X. (2002) Identification of gene expression profile of dorsal root ganglion in the rat peripheral axotomy model of neuropathic pain. Proc. Natl. Acad. Sci. USA. 99: 8360-8365. PDF
C. L. Stucky, M. S. Gold and X. Zhang (2001) Mechanisms of pain. Proc. Natl. Acad. Sci. USA. 98: 11845-11846 PDF
(责任编辑:泉水)
